Objectives
- Define what is a mineral.
- Identify the processes behind the formation of minerals.
- Identify different properties used to identify minerals.
- Classify different minerals according to their composition
What is a Mineral?
Mineral
- It is a naturally-occurring, inorganic solid.
- This means that it cannot be man-made or machine-generated.
- They cannot be a product of living things (eg. coal, fossils)
- This also indicates that they are solid at normal temperature ranges in Earth
- It has orderly crystalline structure that also has a definite chemical composition.
Mineralogy
- study of minerals, their properties, classification, crystallography and ways of distinction.
Formation of Minerals
- Minerals form in one of four major processes:
- Crystallization from Magma
- As molten magma from the depths of the Earth cools, it can form minerals.
- Precipitation
- Substances that are dissolved in water may react to form minerals.
- Pressure/Temperature Changes
- Subtle changes in temperature and pressure can make new minerals form.
- Crystallization from Hydrothermal Solutions
- When some heated solutions touch minerals, chemical reactions may take place and form new minerals.
- Crystallization from Magma
Properties of Minerals
- Minerals have distinct properties.
- These properties can be used to distinguish and identify different minerals
- Luster:
- It describes how light is reflected off the surface of a material.
- It can be classified as metallic and non-metallic.
- Metallic is described as generally opaque and shiny.
- Non-metallic can be described as dull, glassy, resinous, silky, or greasy.
- Color:
- It describes the wavelength of light absorbed and reflected by the crystal.
- They can be an unreliable diagnostic property.
- Impurities within the mineral may give them a different color.
- Streak:
- It describes the color of the crystal when in powder form.
- They can be a better diagnostic property than color.
- Breakage
- This determines how a mineral breaks.
- They can be classified as cleavage and fracture.
- Cleavage is the tendency to create flat surfaces when it breaks.
- Fracture is when a mineral breaks unevenly, irregularly, and non-planar
- Specific Gravity
- It determines how heavy a mineral is.
- It is often referred to its density.
- Crystal Form/Crystal Habit
- It determines the shape of the crystal as they grow.
- It can also describe the layout or arrangement of the atoms inside the mineral.
- Their crystal arrangement can be described as: isometric, tetragonal, orthorhombic, hexagonal, and triclinic
- Isometric is a shape with each face is relatively similar and symmetrical
- Examples are cubic, octahedron, dodecahedron.
- Tetragonal is a shape characterized with a four sided pyramid and a rectangular prism.
- Orthorhombic is a shape with a rectangular prism and a rectangular base.
- Hexagonal has three symmetrical axes that occur in the same plane with the same length.
- Triclinic has three axis where each has different lengths.
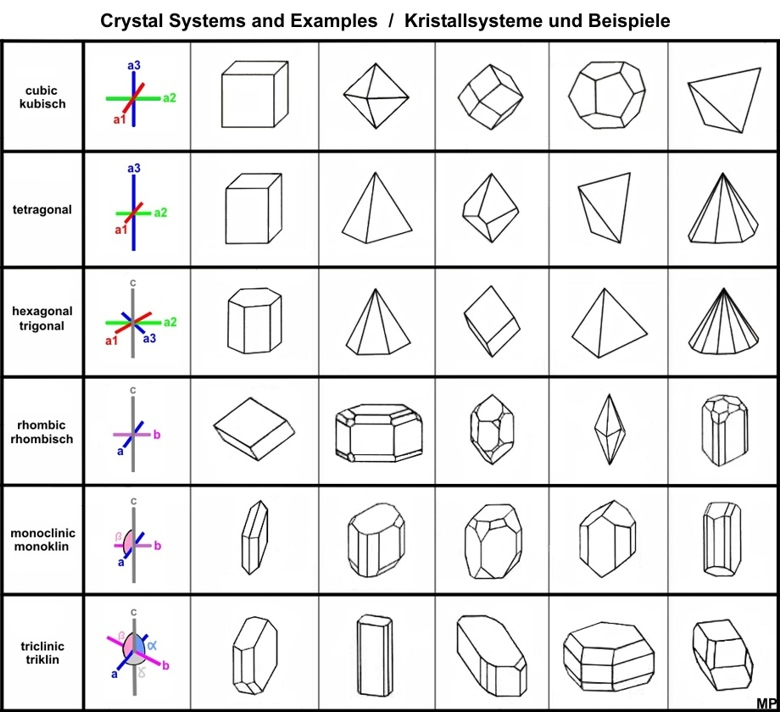
- Isometric is a shape with each face is relatively similar and symmetrical
- Hardness
- It is a measure of the resistance of a mineral to being scratched.
- We test hardness by scratching a mineral against an other material with a known hardness.
- When one scratches the other, then it is harder than the other material
- When they have the same hardness, then both of them scratches one another.
- It is them measured using the Mohs’ Scale.
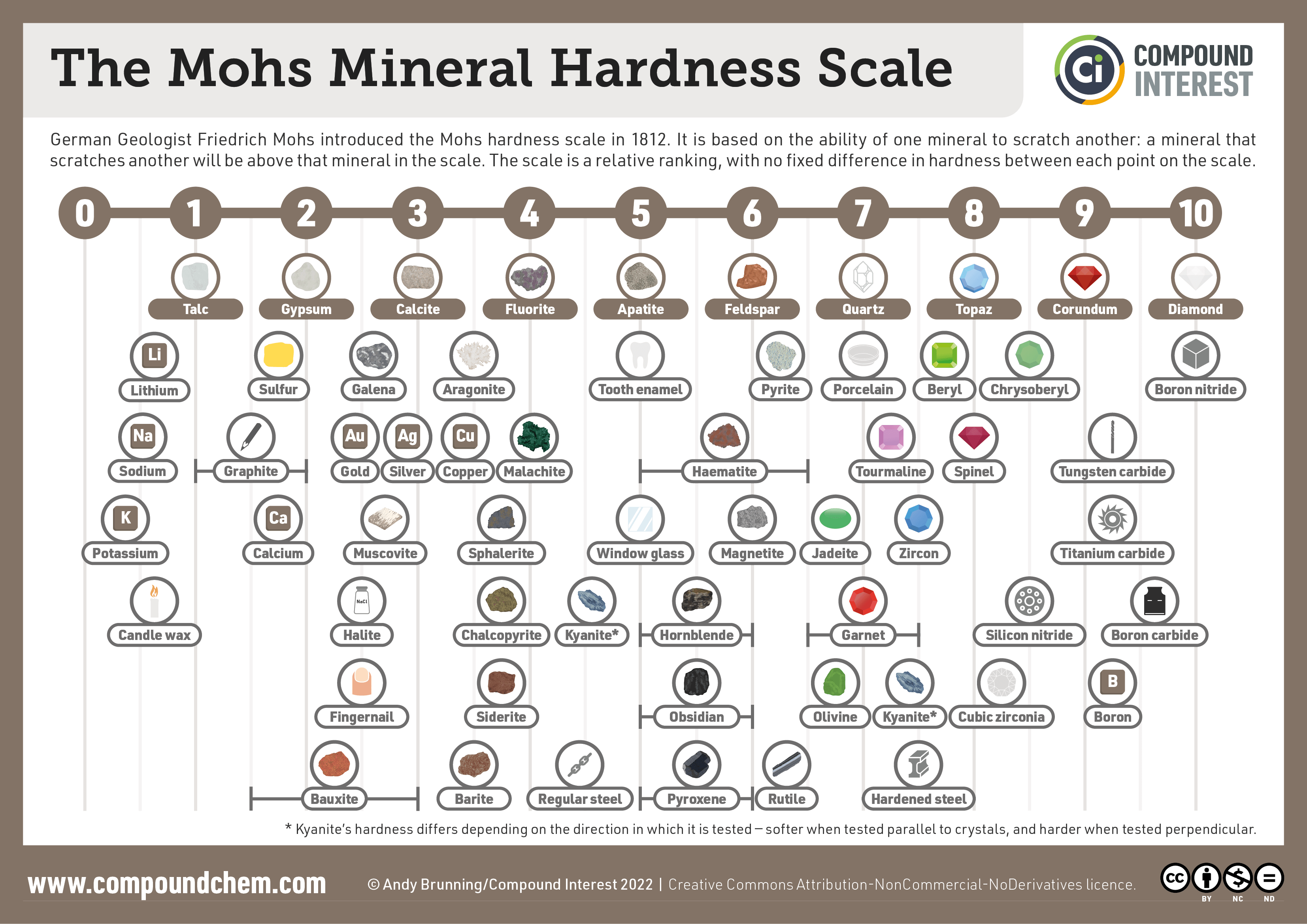
- It is designed by Friedrich Mohs in 1812.
- It consists of minerals arranged from softest to hardest.
- This is where 1 is the softest and 10 is the hardest.
- Other Properties
- Some other unique properties can be used in its identification.
- Some of them includes: magnetism, odor, taste, tenacity, reaction to acid, etc.
- Luster:
Types of Mineral according to Composition
- Minerals can be grouped into two types: silicate and non-silicate minerals
- Silicate Minerals
- They are made of silicon and oxygen.
- They are considered as the most common minerals on Earth.
- 90% of rock-forming minerals belong to this type.
- They usually form by crystallizing cooling magma.
- Non-silicate Minerals
- They are not made of silicon and oxygen.
- They can be classified further into: carbonates, oxides, sulfates, sulfides, halides, and native elements.
- Carbonates
are minerals with carbon, oxygen and one or more other metallic elements - Oxides
are minerals that contain oxygen plus one or more other elements that are usually metals. - Sulfates
are minerals containing a sulfur and oxygen anion plus other ions. - Sulfides
are minerals containing a sulfur anion plus one or more ions - They are also sources of some important metals, such as zinc, copper, or lead.
- Halides are minerals containing a halogen ion plus one or more elements.
- Native Elements are minerals composed of one type of element or atom.
- Can be classified further into three types:
- Metals and Inter-metals have high thermal and electrical conductivity, has metallic luster, and low hardness.
- Semi-metals have lower conductivity and are more fragile than metals.
- Non-metals are non conductive.
- Can be classified further into three types:
- Carbonates
- Silicate Minerals